Enterocele and Massive Vaginal Eversion
Massive vaginal vault prolapse (uterovaginal prolapse) is a devastating condition with discomfort and genitourinary and defecatory abnormalities as the primary consequences. Pelvic organ prolapse is prevalent and associated with significant health-related quality of life and economic consequences.1 References to prolapse of the womb were first made in ancient Egypt, dating back to 1550 BC. Vaginal vault prolapse refers to significant descent of the vaginal apex following a hysterectomy.
Apical prolapse is used to denote prolapse of the vaginal apex with or without the presence of a uterus. Although this obviously is not a new condition, apical prolapse is thought to be increasingly common as life expectancy increases. According to population projections from the US Census Bureau and published age-specific prevalence estimates for bothersome, symptomatic pelvic floor disorders and pelvic organ prolapse, the number of women with uterovaginal prolapse is expected to gradually increase from 3.3 million in 2010 to 4.9 million in 2050.2
Recent histologic and MRI studies of enteroceles, however, have challenged this concept. Studies by Tulikangas et al6 and Hsu et al7 show that a distinct break in the fibromuscular layer denoted as the rectovaginal fascia may not necessarily be present. These studies, however, are small in number and further investigation is warranted. Nevertheless, a careful preoperative and intraoperative search for these defects is often helpful for proper diagnosis.
Normally, posthysterectomy enterocele is precluded by the apposition of pubocervical and rectovaginal fascia (collectively termed endopelvic fascia) at the apex. Anterior, apical, and posterior enteroceles have been described based upon the location of the fascial defect and the location of the ensuing herniation of bowel.
Anterior enteroceles are rare and may occur following sacrospinous ligament fixation, when the proximal vaginal tube is pulled somewhat posteriorly, creating a potential space in the anterior compartment. Because they present as a protrusion of the anterior vaginal wall, they may be the major cause of some apparent cystoceles. In women with an intact uterus, posterior enteroceles have been described. These are due to tearing of the proximal rectovaginal fascia from its attachment to the cardinal-uterosacral ligament complex, which results in descent of the peritoneal contents down the posterior aspect of the vagina.
Prolapse of the vaginal apex may or may not be accompanied by an enterocele. Whereas complete vaginal eversion is obvious, lesser degrees of prolapse and the presence of enterocele are more difficult to discern and require careful evaluation of anterior, posterior, and apical compartment defects. Also, associated functional abnormalities, whether concurrent or potential, must be properly explored, evaluated, and discussed with the patient.
Problem
The International Urogynecological Association and International Continence Society define pelvic organ prolapse as the descent of 1 or more of the anterior vaginal wall, posterior vaginal wall, the uterus (cervix), or the apex of vagina (vaginal vault or cuff scar after hysterectomy).3 Yet, a clear demarcation between normal descent and abnormal prolapse has not been determined. Indeed, a degree of uterine descensus is present in many, if not most, women who are multiparous. Not all patients with prolapse are symptomatic, and the degree of prolapse often does not correlate with the degree of symptoms reported by the patient. Furthermore, pelvic floor-related symptoms do not predict the anatomic location of the prolapse, especially in women with mild-to-moderate prolapse.4
A systematic description of pelvic organ prolapse is useful to help document and communicate the severity of the problem, to establish treatment guidelines, and to improve the quality of research to standardizing definitions.
A systematic description of pelvic organ prolapse is useful to help document and communicate the severity of the problem, to establish treatment guidelines, and to improve the quality of research to standardizing definitions.
The pelvic organ prolapse quantification (POP-Q) has been instituted to address this by defining the extent of prolapse. Specific sites are defined separately on the anterior, posterior, and apical vaginal compartments and are measured with respect to a fixed reference point, the hymen. These measurements can then be categorized into an ordinal staging system ranging from 0-4.
- Stage 0 denotes no prolapse (the apex can descend as far as 2 cm relative to the total vaginal length).
- Stage 1 means that the most distal portion of the prolapse descends to a point more than 1 cm above the hymen.
- Stage 2 denotes that the maximal extent of the prolapse is within 1 cm of the hymen (outside or inside the vagina).
- Stage 3 means that the prolapse extends more than 1 cm beyond the hymen but no more than within 2 cm of the total vaginal length.
- Stage 4 denotes complete eversion, which is defined as extending to within 2 cm of the total vaginal length.
The POP-Q staging system has been validated and demonstrates good interobserver and intraobserver reliability. Although POP-Q staging adequately addresses the extent of prolapse, assumptions about which organ is behind each bulge should be made with caution and should be made only after a complete evaluation.
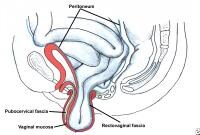
Regarding enterocele, the definition is somewhat more difficult. Previous texts have defined enterocele as a hernia in which peritoneum and abdominal contents displace the vagina and may even be in contact with vaginal mucosa and palpable within the cul-de-sac, as evaluated during an examination in the erect position. A more anatomic definition was proposed by Richardson, who suggested that enterocele occurs when endopelvic fascia does not intervene between the peritoneum and vagina.5
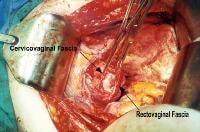
Enterocele and massive vaginal eversion. Large apical endopelvic fascial defect representing an enterocele demonstrated by the transabdominal route. Note the proximal cervicovaginal and rectovaginal fascia separate from the peritoneum.
Recent histologic and MRI studies of enteroceles, however, have challenged this concept. Studies by Tulikangas et al6 and Hsu et al7 show that a distinct break in the fibromuscular layer denoted as the rectovaginal fascia may not necessarily be present. These studies, however, are small in number and further investigation is warranted. Nevertheless, a careful preoperative and intraoperative search for these defects is often helpful for proper diagnosis.
Frequency
Swift reported on the frequency of different stages of pelvic organ prolapse based upon the POP-Q staging system.8 In a routine gynecologic clinic population, most women had stage 1 or stage 2 prolapse (43.3% and 47.7%, respectively), few women had stage 0 or stage 3 prolapse (6.4% and 2.6%, respectively), and no women had stage 4 pelvic organ prolapse. Samuelsson et al report a prevalence of 30.8% for any prolapse, using the Baden-Walker halfway system, in a study of the general population in Sweden.9 Vaginal vault prolapse is thought to occur postoperatively in 0.5% of hysterectomy cases, whether they are performed vaginally or abdominally.
In a population-based Dutch study, the prevalence of pelvic organ prolapse by POP-Q staging was as follows: Stage 0 = 25.0%, stage I = 36.5%, stage II = 33%, stage III = 5.0%, and stage IV = 0.5%.10
In a population-based Dutch study, the prevalence of pelvic organ prolapse by POP-Q staging was as follows: Stage 0 = 25.0%, stage I = 36.5%, stage II = 33%, stage III = 5.0%, and stage IV = 0.5%.10
Etiology
Swift reported that significant trends for increasing prolapse were found with advancing age, parity, postmenopausal status, previous hysterectomy, and prior corrective surgery for prolapse.8 Multivariate analysis in a study performed by Samuelsson et al revealed independent statistical associations with age, parity, maximal birth weight, and pelvic floor muscle strength.9 Such associations were not found regarding the weight or hysterectomy status.
Recent epidemiological studies by Dietz et al10 and Slieker-ten Hove et al11 contradict the opinion that female pelvic organ prolapse worsens with age. Furthermore, Sze et al demonstrated that vaginal birth is not associated with POP-Q stages III and IV but is associated with an increase in POP-Q stage II prolapse.12
Racial differences have been reported for pelvic organ prolapse, although it is not yet clear whether the differences are biological or sociocultural. Whitcomb et al showed that compared with African-American women, Latina and white women had 4-5 times higher risk of symptomatic prolapse, and white women had 1.4-fold higher risk of objective prolapse at or beyond the hymen.1
Racial differences have been reported for pelvic organ prolapse, although it is not yet clear whether the differences are biological or sociocultural. Whitcomb et al showed that compared with African-American women, Latina and white women had 4-5 times higher risk of symptomatic prolapse, and white women had 1.4-fold higher risk of objective prolapse at or beyond the hymen.1
Pathophysiology
Genetic factors may play a significant role in the development of uterovaginal prolapse. Allen-Brady et al found linkage evidence for a recessive predisposition gene for pelvic floor disorders on chromosome 9q.13
Current basic science research suggests a molecular etiology of pelvic organ prolapse. Some studies demonstrate an increased rate of apoptosis and significant depletion of mitochondrial DNA in uterosacral ligaments in women with uterovaginal eversion.14,15
The precise etiology regarding pelvic organ prolapse remains elusive. Additional theories include diminished sacral nerve function and/or defects in collagen.
The pelvic floor is a unique and complex system constructed of striated muscles, support and suspensory ligaments, fascial layers, and an intricate neural network. When this system is damaged, pelvic floor failure occurs and pelvic organ prolapse ensues.16
DeLancey describes the anatomy of vaginal vault prolapse in terms of 3 levels of support.17
Current basic science research suggests a molecular etiology of pelvic organ prolapse. Some studies demonstrate an increased rate of apoptosis and significant depletion of mitochondrial DNA in uterosacral ligaments in women with uterovaginal eversion.14,15
The precise etiology regarding pelvic organ prolapse remains elusive. Additional theories include diminished sacral nerve function and/or defects in collagen.
The pelvic floor is a unique and complex system constructed of striated muscles, support and suspensory ligaments, fascial layers, and an intricate neural network. When this system is damaged, pelvic floor failure occurs and pelvic organ prolapse ensues.16
DeLancey describes the anatomy of vaginal vault prolapse in terms of 3 levels of support.17
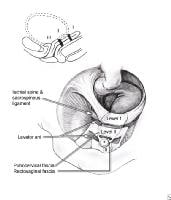
Enterocele and massive vaginal eversion. Levels of support as described by DeLancey (1992). Note that level I refers to apical (or uterovaginal) support.
- Level I involves the support of the upper vagina and cervix or the vaginal cuff (in a woman who has undergone total hysterectomy) by the cardinal-uterosacral ligament complex.
- Level II denotes the lateral support of the mid vagina to the arcus tendineus fascia pelvis (white line).
- Level III is represented by the fusion of tissue along the base of the urethra and the distal rectovaginal septum to the perineal body.
The conditions of enterocele and vaginal eversion represent failures of level I support, although other compartments may be affected. Uterovaginal prolapse does not denote intrinsic uterine disease and, therefore, may not necessarily require a hysterectomy in all cases. It should be noted, however, that no evidence proves or disproves the benefit of hysterectomy at the time of apical suspension.
Apical prolapse occurs because of tearing or attenuation of the cardinal-uterosacral ligament complex. This results in failure to support the upper vagina and/or uterus over the pelvic diaphragm, which should be in a near-horizontal plane in a woman in the erect position. Level I support is considered most important in maintaining adequate overall pelvic support.
Richardson describes an enterocele in anatomic terms, as a break in the integrity of endopelvic fascia at the vaginal apex.18
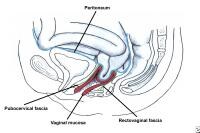
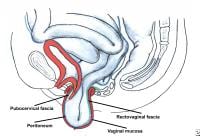
Enterocele and massive vaginal eversion. Normal posthysterectomy vaginal vault. Note the presence of continuity of the endopelvic fascia at the vaginal apex, resulting from the fusion of cervicovaginal and rectovaginal fascia, and their fusion with the uterosacral ligament portion of endopelvic fascia.
Normally, posthysterectomy enterocele is precluded by the apposition of pubocervical and rectovaginal fascia (collectively termed endopelvic fascia) at the apex. Anterior, apical, and posterior enteroceles have been described based upon the location of the fascial defect and the location of the ensuing herniation of bowel.
Apical enterocele is the most common enterocele and, by definition, can develop only after a hysterectomy. Apical enterocele may present with or without vaginal vault prolapse.
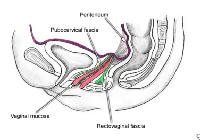
Enterocele and massive vaginal eversion. Early enterocele with no vault prolapse. Note contact of peritoneal contents with vaginal mucosa, with no intervening endopelvic fascia.
Enterocele and massive vaginal eversion. Progressive enterocele now demonstrating true vaginal vault prolapse.
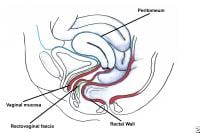
Anterior enteroceles are rare and may occur following sacrospinous ligament fixation, when the proximal vaginal tube is pulled somewhat posteriorly, creating a potential space in the anterior compartment. Because they present as a protrusion of the anterior vaginal wall, they may be the major cause of some apparent cystoceles. In women with an intact uterus, posterior enteroceles have been described. These are due to tearing of the proximal rectovaginal fascia from its attachment to the cardinal-uterosacral ligament complex, which results in descent of the peritoneal contents down the posterior aspect of the vagina.
Enterocele and massive vaginal eversion. Posterior enterocele in a patient with a uterus. Note that peritoneal contents have dissected between the vaginal mucosa and rectovaginal fascia through a proximal defect.
Posterior enterocele is usually accompanied by significant uterovaginal prolapse and prolapse of other compartments as well.
Recently, histologic studies by Tulikangas et al failed to find breaks in the fibromuscular layer in women who underwent surgical correction for enterocele compared with controls (women who did not have pelvic organ prolapse).6 Admittedly, the authors' findings did not correlate with their subjective clinical findings of thinning of the vaginal wall in enteroceles. Hsu et al similarly found a lack of difference in vaginal wall thickness on MRI studies of patients with prolapse and their normal controls.7 The numbers in these studies, however, were small and further investigation is needed before this controversy is fully resolved.
Presentation
Patients may present with an obvious vaginal bulge that is visualized or felt by the patient. Conversely, the patient may report a vague sense of pelvic heaviness or a sensation that something is about to fall out. The bulging is often noted to be worse toward the end of the day, as compared with when the patient first wakes up, or when the patient is straining at defecation or urination. When vaginal epithelium remains exteriorized, it undergoes cornification and, often, ulceration, which can result in significant pain and infection.
Functional difficulties may be encountered during coitus. Defecation may be difficult; associated constipation is very common. Incomplete bladder emptying also is common, and, in severe cases, complete obstruction may be observed. Voiding dysfunction may result in frequent urinary tract infections and, occasionally, overflow incontinence. Due to kinking of the urethra, occult (potential) stress incontinence and even intrinsic sphincter deficiency may be present. A history of stress incontinence that spontaneously improved and/or resolved as the prolapse progressively worsened is especially concerning for the presence of occult stress incontinence.
A cross-sectional study indicates that urge urinary incontinence is associated with anterior wall prolapse, while stress urinary incontinence is strongly linked to posterior wall prolapse.4 Severe pelvic prolapse may result in ureteral kinking, with the potential for hydroureter, hydronephrosis, and subsequent renal damage. Hydronephrosis occurred with an overall prevalence of 7.7%, with severe hydronephrosis occurring in 0.9%. Hydronephrosis severity was proportional to prolapse severity with a higher likelihood of uterovaginal versus posthysterectomy vaginal prolapse.19
A cross-sectional study indicates that urge urinary incontinence is associated with anterior wall prolapse, while stress urinary incontinence is strongly linked to posterior wall prolapse.4 Severe pelvic prolapse may result in ureteral kinking, with the potential for hydroureter, hydronephrosis, and subsequent renal damage. Hydronephrosis occurred with an overall prevalence of 7.7%, with severe hydronephrosis occurring in 0.9%. Hydronephrosis severity was proportional to prolapse severity with a higher likelihood of uterovaginal versus posthysterectomy vaginal prolapse.19
A detailed history is required to evaluate the patient. Information regarding any functional problems that may be caused by the prolapse should be ascertained. Essential to the preoperative evaluation and surgical decision-making is the review of any prior pelvic surgery, including obtaining operative reports, especially if surgery was performed for prior pelvic floor dysfunction.
A commitment to treat all associated pelvic floor defects requires a careful and comprehensive urogynecologic examination. A diligent search for all pelvic support defects and repair of significant defects increases the likelihood for surgical success. The apical, anterior, and posterior compartments are evaluated separately, with and without straining and/or coughing in the supine position and again in the erect position, if needed, preferably with an empty bladder. The POP-Q exam is helpful for quantifying the extent of prolapse and accurate follow-up. Carefully evaluate the rectovaginal septum for integrity, strength, and thickness along its entire length. Look for any signs of enterocele, such as bowel peristalsis, along the posterior vagina or near the apex. Look for any obvious pubocervical/rectovaginal detachments at the periphery of an apical bulge. Evaluate the cul-de-sac in the supine and standing positions, with and without Valsalva maneuvers.
Indications
Treatment of pelvic organ prolapse is indicated if it is symptomatic or is causing associated morbidity. Asymptomatic prolapse, with minor degrees of protrusion that cause no other problems, must be discussed with the patient but does not necessarily require treatment.
In the older population, even extensive prolapse may be asymptomatic from the patient's point of view, but questioning her family or caregiver may reveal troublesome symptoms, and further evaluation may reveal significant resultant morbidity.
Offer conservative management to these patients as the initial management option. Conservative management may include observation with mild degrees of asymptomatic prolapse or a pessary fitting. Surgical management may be considered in appropriate candidates if conservative therapies fail or are declined by the patient.
Relevant Anatomy
The cardinal-uterosacral ligaments are localized thickenings of the endopelvic fascia that invest the pelvic organs. The same endopelvic fascia that is anterior to the vagina is called pubocervical; posteriorly, it is termed rectovaginal fascia or Denonvillier fascia. Laterally, the endopelvic fascia attaches the vagina to the arcus tendineus fascia pelvis to provide lateral support to the mid vaginal compartment, whereas the distal rectovaginal fascia attaches laterally to the aponeurosis of levator ani.20
The integrity of the vaginal apex following hysterectomy depends on the fusion of the pubocervical fascia with the rectovaginal fascia. Surgically, the uterosacral ligaments lie medial to the ureters in the pelvis. The proximal uterosacral ligament fans out and attaches to the lateral aspect of the sacrum. MRI studies show slight variations in the attachment of the uterosacral ligament, although most overlay the sacrospinous ligament/coccygeus muscle. The proximal vagina usually points into the hollow of the sacrum towards sacral levels 3 and 4 (S3, S4) and maintains a near-horizontal plane when the woman stands erect.
Although the term fascia is frequently used to denote the surgically significant layer used for pelvic reconstruction, histologically, it is a fibromuscular layer with varying amounts of smooth muscle, collagen, and elastin that is located deep to the epithelium.
Contraindications
Pessary use is contraindicated in the presence of vaginal ulceration and breakdown or in the presence of an active vaginal infection. Severe vaginal atrophy is best treated prior to starting pessary use, in the absence of contraindications for estrogen use.
The evaluation of a patient for surgical repair is a topic that is too broad for this article. However, one should tailor the proposed operation to the specific defects noted preoperatively, taking into consideration the patient's overall health and prior surgical history. The chosen approach, whether vaginal, abdominal, laparoscopic, or robotically assisted should be selected with careful consideration of these patient-related points, in addition to the surgeon's level of skill and available local resources. Appropriate consultations and referrals during the preoperative evaluation can ensure the highest degree of success and safety.












i love online doctors sites..you are doing a great job dear...
ReplyDelete.........................................
Reversal of Tubal Ligation